I linked to a systematic review of Ivermectin earlier. The benefits in legitimate studies are being seen in parts of the world with high levels of underlying parasitic infection. The benefit doesn't show up when analyzing and comparing the studies done in the developed world.
Hydroxychloroquine
14,165 Views |
124 Replies |
Last: 3 yr ago by Zobel
You don't have to review them all. Reviewing these kind of things takes a fair amount of skill and knowledge. Read this, someone has done it for you.
https://astralcodexten.substack.com/p/ivermectin-much-more-than-you-wanted
As far as ivermectin goes, here's the graph you need to see.
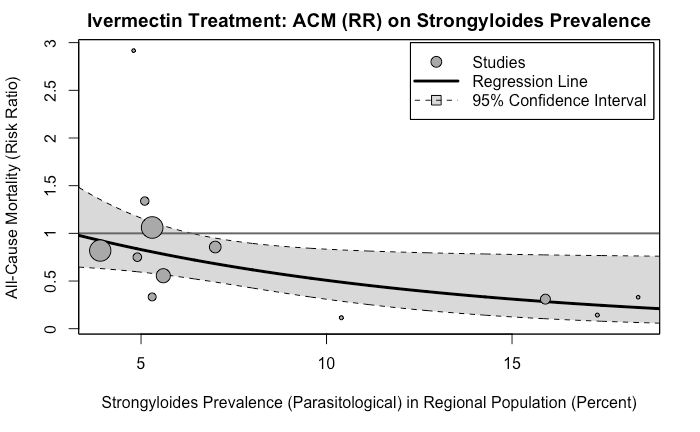 There's a significant confounder at play here - worms. Worms are a comorbidity for covid, and the places that have higher worms prevalence showed better results with ivermectin...because it kills the worms. Just like the in vitro effect of HCQ and azithromycin was confounded by phospholipidosis. Because medicine is hard.
There's a significant confounder at play here - worms. Worms are a comorbidity for covid, and the places that have higher worms prevalence showed better results with ivermectin...because it kills the worms. Just like the in vitro effect of HCQ and azithromycin was confounded by phospholipidosis. Because medicine is hard.
https://astralcodexten.substack.com/p/ivermectin-much-more-than-you-wanted
As far as ivermectin goes, here's the graph you need to see.
 There's a significant confounder at play here - worms. Worms are a comorbidity for covid, and the places that have higher worms prevalence showed better results with ivermectin...because it kills the worms. Just like the in vitro effect of HCQ and azithromycin was confounded by phospholipidosis. Because medicine is hard.
There's a significant confounder at play here - worms. Worms are a comorbidity for covid, and the places that have higher worms prevalence showed better results with ivermectin...because it kills the worms. Just like the in vitro effect of HCQ and azithromycin was confounded by phospholipidosis. Because medicine is hard.But there is a mechanism. You seem wholly uniformed on the matter if you are unaware of the mechanism. Did you just think a bunch of scientist thought dewormer would work as an antiviral magically?Derrida said:The more experienced and better researchers have shown it doesn't work. The poorly constructed retrospective studies suggest yes and no.NicosMachine said:To dismiss the use various therapeutics as reliance on "one small, meaningless, study" is woefully ignorant or purposefully misleading. There are thousands of doctors prescribing Hydroxychloroquine or Ivermectin as an early therapeutic for Covid and who will testify as to the anecdotal efficacy. They base their use of those drugs on both common sense (Ivermectin has been shown to have antiviral properties both in vitro and in vivo with other viruses) and their personal experience. Here is a summary of some of the studies on Ivermectin.Windy City Ag said:Unfortunately our society has the process backwards. We start with a gut feeling that makes us happy and then try to find the data that fits our narrative and generally hide or argue away contrary evidence.Quote:
I'll take that as a no. I don't rely on this board for junk data. It was created for accurate, real information for covid and to provide a forum for medical professionals giving guidance. Save the conspiracies and junk science for another place. That's what f16 is for.
No matter how many times weak or contradictory evidence sprouts up, folks are going to be dug in on their particular stance and wave around one small, meaningless study to make their point.
https://ivmmeta.com
I have not reviewed all the studies and I'm also familiar with studies which indicate Ivermectin has no therapeutic effects for Covid. I don't know the answer, but to dismiss other scientists and actual doctors who are treating thousands of patients as some sort of hacks or rubes seems quite arrogant.
If people want to subject themselves to the risks, fine, but without a mechanism, no reason to believe it can work.
https://link.springer.com/content/pdf/10.1007/s00210-020-01902-5.pdf
The inadequacy of a proposed MoA and the challenges associated with achieving this kind of effect in a clinical setting has already been laid out for you. It's not enough. Every drug that goes to human clinical trials has a promising in vitro effect or proposed MoA. That's why they think it's worth it to try them. And even so, 90%+ of drugs that go to clinical trails fail, because medicine is hard.
Failures can indeed arise from a lack of efficacy. But there are many other reasons drugs "fail" clinical trials - there are issues with safety, or a lack of funding to complete a trial, as well as other factors such as failing to maintain good manufacturing protocols, failing to follow FDA guidance, or problems with patient recruitment, enrollment, and retention.Zobel said:
The inadequacy of a proposed MoA and the challenges associated with achieving this kind of effect in a clinical setting has already been laid out for you. It's not enough. Every drug that goes to human clinical trials has a promising in vitro effect or proposed MoA. That's why they think it's worth it to try them. And even so, 90%+ of drugs that go to clinical trails fail, because medicine is hard.
Only 57% fail due to "efficacy" and even then, there are many reasons that potentially efficacious drugs can still fail to demonstrate efficacy, including a flawed study design, an inappropriate statistical endpoint, or simply having an underpowered clinical trial (i.e., sample size too small to reject the null hypothesis), which may result from patient dropouts and insufficient enrollment.
There are far more studies indicating efficacy and safety of Ivermectin as a potential Covid therapeutic than those that indicate otherwise. To blithely dismiss the overwhelming majority of studies without reference to some methodological flaw because there are a few contradictory studies seems unscientific, especially in light of the success reported by actual treating physicians. I don't care one way or the other, but find the certainty with which laypersons dismiss such evidence amusing.
Thank you. I'll take a closer look. On first perusal, the author definitely makes a point I've stressed on this board time and again:Zobel said:
You don't have to review them all. Reviewing these kind of things takes a fair amount of skill and knowledge. Read this, someone has done it for you.
https://astralcodexten.substack.com/p/ivermectin-much-more-than-you-wanted
As far as ivermectin goes, here's the graph you need to see.There's a significant confounder at play here - worms. Worms are a comorbidity for covid, and the places that have higher worms prevalence showed better results with ivermectin...because it kills the worms. Just like the in vitro effect of HCQ and azithromycin was confounded by phospholipidosis. Because medicine is hard.
So what do you do?
This is one of the toughest questions in medicine. It comes up again and again. You have some drug. You read some studies. Again and again, more people are surviving (or avoiding complications) when they get the drug. It's a pattern strong enough to common-sensically notice. But there isn't an undeniable, unbreachable fortress of evidence. The drug is really safe and doesn't have a lot of side effects. So do you give it to your patients? Do you take it yourself?
Here this question is especially tough, because, uh, if you say anything in favor of ivermectin you will be cast out of civilization and thrown into the circle of social hell reserved for Klan members and 1/6 insurrectionists. All the health officials in the world will shout "horse dewormer!" at you and compare you to Josef Mengele. But good doctors aren't supposed to care about such things. Your only goal is to save your patient. Nothing else matters.
You should cite your sources when you copy pasta. Like this one.
https://daneshyari.com/article/preview/8519212.pdf
As for ivermectin, you're simply not correct. Many of the trials were out-and-out fraudulent, some probably never even happened. Most had some kind of complicated endpoint, versus a cut and dry benefit (like fewer deaths).
The author of that paper is a psychiatrist and the blog covers a lot of topics, but medical research is one of them. If you're interested in that kind of thing, read up. And his conclusion is that ivermectin doesn't work absent parasite comorbidity. In other words, it doesn't do anything for covid per se.
https://daneshyari.com/article/preview/8519212.pdf
As for ivermectin, you're simply not correct. Many of the trials were out-and-out fraudulent, some probably never even happened. Most had some kind of complicated endpoint, versus a cut and dry benefit (like fewer deaths).
The author of that paper is a psychiatrist and the blog covers a lot of topics, but medical research is one of them. If you're interested in that kind of thing, read up. And his conclusion is that ivermectin doesn't work absent parasite comorbidity. In other words, it doesn't do anything for covid per se.
Sapper Redux said:01agtx said:
It's ridiculous to compare goat piss and a bacterial infection, something no one does, to MDs who are having success with a protocol.
I'm going over the top to make a point. Success with a protocol that's not properly tested doesn't mean the protocol works. It means you have success while using a protocol. Correlation does not equal causation. That's why we have research and regulations.
Maybe I just don't understand your line of thinking. There are doctors that continue to use protocols that worked in 2020. Would you have had them stop using that protocol because a study came out that said that one piece of their protocol doesn't work? What would you suggest doing instead? I think we are well beyond the correlation doesn't equal causation argument.
Pfizer's Comirnaty is the only vaccine given full FDA approval. It is not available in the United States? Why is that?Zobel said:
The mRNA vaccines have undergone multiple RCTs with thousands of participants - much larger sizes than normal. The results of those trials were published - you can read them here and here for Pfizer and Moderna's Phase III, respectively. You will not find a similar RCT for ivermectin or HCQ, much less one with such clear outcomes.
Pfizer's vaccine has full FDA approval. Moderna's does not, but not because of not meeting any evidence-based approach of medicine. The FDA gets 10 months to review their application, and has said that they will make a decision on Moderna in January of 2022. The delay is to get more information on the risk of myocarditis in young men.
All medicines have side effects and none are perfectly effective. It's an error to make an equivalence between drugs based on that alone. Using an undefined standard for 'evidence' to draw a comparison is a further error.
No one is claiming perfect certainty. You have varying degrees of evidence, and varying degrees of efficacy. HCQ and Ivermectin come nowhere close to the standard of evidence and efficacy that the vaccines do. In most cases they've failed to show evidence vs placebo in RCTs.
That seems quite confusing. Does that mean that it's something other than Comirnaty being given to kids under 16? What about boosters, are those "Pfizer" or "Comirnaty"? If they are exactly the same, why the silly name usage depending on your age or whether its a booster?Zobel said:
Because it is? Comirnaty is just the brand name.
https://www.usatoday.com/story/news/factcheck/2021/10/20/fact-check-comirnaty-pfizers-fda-approved-vaccine-available-us/8538861002/?fbclid=IwAR0ARf47_DdiiMioMlrAmR9bcjY7RKV7AhDWKczfOZlBVATK_FxbJ3jzkNk
https://ivmmeta.com/#strongyloidesZobel said:
You don't have to review them all. Reviewing these kind of things takes a fair amount of skill and knowledge. Read this, someone has done it for you.
https://astralcodexten.substack.com/p/ivermectin-much-more-than-you-wanted
As far as ivermectin goes, here's the graph you need to see.There's a significant confounder at play here - worms. Worms are a comorbidity for covid, and the places that have higher worms prevalence showed better results with ivermectin...because it kills the worms. Just like the in vitro effect of HCQ and azithromycin was confounded by phospholipidosis. Because medicine is hard.
Because they're not allowed to market it unless it has fda approval. They can market it as comirnaty, but only for fda approved use(s). It's all the same stuff in the vials.
So do the vials given as boosters and to those under 16 not say "comirnaty" and the vials given to everyone else say "comirnaty"? Comirnaty is not approved as a booster or for those under 16 so that must be the case and seems rather unscientific.Zobel said:
Because they're not allowed to market it unless it has fda approval. They can market it as comirnaty, but only for fda approved use(s). It's all the same stuff in the vials.
Haha I don't think anyone has ever accused the USG of being scientific. It's a bureaucratic/ regulatory thing.
Zobel said:
Because it is? Comirnaty is just the brand name.
https://www.usatoday.com/story/news/factcheck/2021/10/20/fact-check-comirnaty-pfizers-fda-approved-vaccine-available-us/8538861002/?fbclid=IwAR0ARf47_DdiiMioMlrAmR9bcjY7RKV7AhDWKczfOZlBVATK_FxbJ3jzkNk
Is this no longer accurate?
"COMINARTY products are not orderable at this time."
https://www.cdc.gov/vaccines/programs/iis/COVID-19-related-codes.html
"The FDA-approved Comirnaty (COVID-19 Vaccine, mRNA) and the two EUA authorized formulations of Pfizer-BioNTech COVID-19 Vaccine for ages 12 years and older, when prepared according to their respective instructions for use, can be used interchangeably without presenting any safety or effectiveness concerns. The products are legally distinct with certain differences that do not impact safety or effectiveness."
https://www.fda.gov/vaccines-blood-biologics/qa-comirnaty-covid-19-vaccine-mrna
Why wouldn't they give full approval to the Pfizer vaccine at the same time? Does it have to do with liability?
"Because all vaccines that are recommended, officially recommended for children get liability protection, even if an adult gets that vaccine."
https://www.thegatewaypundit.com/2021/12/robert-f-kennedy-jr-explains-going-kids-not-think-video/
The CDC link is about labeling and registry of the product under the FDA guidelines. All drugs in the US have to be registered with the FDA under a National Drug Code (NDC). Before full approval they had an NDC for the drug. After full approval they created two new NDCs. One of the new NDCs is for 25 multiple does vials (0069-1000-03) and the other is for 195 multiple dose vials (0069-1000-02) as per the insert here.
Since the EUA drug and Comirnaty are the same, and some uses of the drug aren't full approved - all the uses still under EUA - they are still making them without the Comirnaty label so they don't produce product which can't be used for regulator reasons. Comirnaty is only for 16+, the emergency use authorization is for 5 and older, the third shot for 12 and older, and booster for 16 and older per the FDA here.
I don't understand this question. The FDA approved the Pfizer vaccine. That's what Comirnaty is. But approval is only for certain uses. Once a product is approved, physicians are allowed to prescribe if off-label, but it can't be marketed for off-label use. It's illegal.
So the drug has full approval for some uses (two shot vaccine for ages 16+) but emergency use and not approval for not others (booster, for kids). Comirnaty is only for the former, not the latter - legally. The product inside is the same.
Robert F Kennedy Jr is an anti-vax nut, and you should probably ignore everything he says.
Here's a Q&A on this topic directly from back in August.
https://www.nebraskamed.com/COVID/you-asked-we-answered-are-pfizers-comirnaty-and-biontech-covid-19-vaccines-the-same-or-different
Since the EUA drug and Comirnaty are the same, and some uses of the drug aren't full approved - all the uses still under EUA - they are still making them without the Comirnaty label so they don't produce product which can't be used for regulator reasons. Comirnaty is only for 16+, the emergency use authorization is for 5 and older, the third shot for 12 and older, and booster for 16 and older per the FDA here.
Quote:
Why wouldn't they give full approval to the Pfizer vaccine at the same time? Does it have to do with liability?
I don't understand this question. The FDA approved the Pfizer vaccine. That's what Comirnaty is. But approval is only for certain uses. Once a product is approved, physicians are allowed to prescribe if off-label, but it can't be marketed for off-label use. It's illegal.
So the drug has full approval for some uses (two shot vaccine for ages 16+) but emergency use and not approval for not others (booster, for kids). Comirnaty is only for the former, not the latter - legally. The product inside is the same.
Robert F Kennedy Jr is an anti-vax nut, and you should probably ignore everything he says.
Here's a Q&A on this topic directly from back in August.
https://www.nebraskamed.com/COVID/you-asked-we-answered-are-pfizers-comirnaty-and-biontech-covid-19-vaccines-the-same-or-different
Thanks for taking the time to share information about Comirnaty with links. I have felt similarly about RFK in the past, but I'm looking forward to reading his book on Fauci.
Your response was well-documented and may be correct. The sources were appreciated. I happened to catch Dr Malone on Rogan on Sunday. His credentials are impressive and the most relevant are covered around the 6:30 mark.
https://odysee.com/@NoFriendRequests:5/joerogan-robertmalone:c
The video was followed up with an interview with Bannon the next day or two, in which he answered my questions (in Part 2 at 4:15 mark), but his response differs from yours.
https://warroom.org/2022/01/03/dr-malone-medical-lies-are-result-of-mass-formation-psychosis/
I came across this case that should hopefully resolve the confusion. Ironically, it was filed by RFK.
https://cdn.locals.com/documents/47656/47656_h9iscg4r4q4qflx.pdf
"However, FDA's little caveat was buried in footnote number 8 - Comirnaty was unavailable to the public. (Ftn. 8 Exh. 19-1 Page 29 of 116) The 'approved' drug was, and continues to be, unmanufactured and unable to be distributed to the populace en masse…Defendants demand this Court accept the same fiction they propagated - that two different vaccines are a single vaccine - 'identical' but 'legally distinct,' but ignore their own internal documents showing critical 'certain differences,' including different manufacturing rules, different labeling rules, different companies approved, and, critically, completely different legal status."
This was the case that changed my thinking about him.
https://www.icandecide.org/ican_lawsuits/the-food-and-drug-administration-fda-admits-it-has-never-licensed-any-influenza-vaccine-for-use-by-pregnant-women-and-does-not-have-a-single-trial-supporting-the-safety-of-this-practice/
https://odysee.com/@NoFriendRequests:5/joerogan-robertmalone:c
The video was followed up with an interview with Bannon the next day or two, in which he answered my questions (in Part 2 at 4:15 mark), but his response differs from yours.
https://warroom.org/2022/01/03/dr-malone-medical-lies-are-result-of-mass-formation-psychosis/
I came across this case that should hopefully resolve the confusion. Ironically, it was filed by RFK.
https://cdn.locals.com/documents/47656/47656_h9iscg4r4q4qflx.pdf
"However, FDA's little caveat was buried in footnote number 8 - Comirnaty was unavailable to the public. (Ftn. 8 Exh. 19-1 Page 29 of 116) The 'approved' drug was, and continues to be, unmanufactured and unable to be distributed to the populace en masse…Defendants demand this Court accept the same fiction they propagated - that two different vaccines are a single vaccine - 'identical' but 'legally distinct,' but ignore their own internal documents showing critical 'certain differences,' including different manufacturing rules, different labeling rules, different companies approved, and, critically, completely different legal status."
This was the case that changed my thinking about him.
https://www.icandecide.org/ican_lawsuits/the-food-and-drug-administration-fda-admits-it-has-never-licensed-any-influenza-vaccine-for-use-by-pregnant-women-and-does-not-have-a-single-trial-supporting-the-safety-of-this-practice/
Anyone can say anything in a lawsuit document like that. Again, you can probably safely ignore anything that comes from RFK.
Featured Stories
See All
Scouting Report: No. 10 Texas A&M at No. 17 Oklahoma
by Tom Schuberth
A&M's growing 2026 class features pledges from across the country
by Ryan Brauninger
8:10
10h ago
882
7:00
10h ago
3.6k
Members of A&M's 2025 signing class officially make Aggieland home
by Ryan Brauninger

